Abstract
Introduction
In utero hematopoietic cell transplantation (IUHCT) of allogenic bone marrow mononuclear cells (MNC) results in successful engraftment across immune barriers. This is secondary to immune tolerance that is acquired due to the presence of donor cells in the thymus prior to E17. We have demonstrated that immune tolerance to specific proteins is possible by injecting nanoparticle encapsulated peptides which are taken up by dendritic cells and presented in the thymus.
The aim of this study is to show that in utero injections of nanoparticle encapsulated antigen can produce tolerance to that antigen. We demonstrate this by preventing the autoimmune disease Experimental Autoimmune Encephalopathy (EAE, the mouse equivalent of multiple sclerosis) by injecting NPs loaded with the peptide Myelin Oligodendrocyte Glycoprotein (35-55) (MOG35-55.)
Methods
In utero injections of nanoparticles were performed in C57BL/6 mice at E14 intravenously through the vitelline vein. Post-natal boosters were injected subcutaneously at 2, 4, 6, and 8 weeks of age. Disease inducing injections of MOG with Complete Freud's Adjuvant (CFA) was given at 8 1/7 weeks. Intraperitoneal pertussis toxin (PT) was given on the same day as the MOG/CFA and again 48 hours later. Positive controls got MOG/CFA and PT injections. Negative controls got CFA without MOG as well as the PT injections.
Mice were monitored for paralysis using the 5-point EAE scoring system for 4 weeks after the MOG/CFA injection. Spines and brains were harvested 2 weeks after the disease inducing MOG/CFA injection. Spines and brains were filtered and then MNCs were isolated using a Percoll gradient. MNCs were stained for CD45, CD19, CD11b, CD3, CD4, CD8, and I-A(b) MOG35-55 tetramer. Cells were then analyzed on the FACSAria™.
Results are expressed as mean±SEM, and statistical analysis was performed using 1-way ANOVA with Bonferroni post-hoc tests. Experimental protocols were approved by the Institutional Animal Care and Use Committee at The Children's Hospital of Philadelphia.
Results
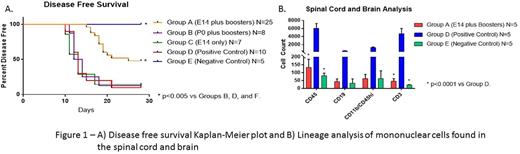
Mice that received MOG nanoparticles prenatally along with boosters (Group A) developed disease with less frequency than mice that received MOG nanoparticles postnatally along with boosters (Group B) or positive controls (Group D) (52.0% vs 87.5% vs 90.0% p<0.005) supporting the theory that antigen needs to be expressed prior to E17 in the thymus to develop tolerance. Mice that were injected prenatally with MOG nanoparticles and did not receive booster injections (Group C) also did as poorly as positive controls (85.72% vs 90.0%) suggesting that continued antigen expression is necessary to maintain tolerance. Group A also had significantly lower EAE scores on days 15 to 28 compared to Group B, Group C, and the positive control group (p<0.005).
Spinal cord and brain analysis of the Group A mice showed significantly fewer CD45 cells compared to the positive control but not the negative control (133.2±52.0 vs 6062.5±1166.9 vs 79.8±15.0 p<0.0001.) The same is seen with CD3 cells (45.6±17.0 vs 4727.3±1283.0 vs 21.3±2.8 p<0.0001) and CD4 cells (12.6±5.7 vs 3339.6912.1 vs 11.8±0.8 p<0.0001.) Finally, the same is seen with CD4+ Tetramer+ cells (0.7±0.3 vs 379.1±156.6 vs 2.9±1.0 p<0.0001) showing that there is central tolerance to the MOG peptide.
Conclusion
By decreasing the frequency and average severity of EAE and demonstrating the lack of CD4+ Tetramer+ cells in the spine, we have shown that autoimmune disease can be prevented by central tolerance to the target antigen. We have demonstrated that this central tolerance is possible with prenatal injections of nanoparticle encapsulated peptide. We have also shown that continued antigen presentation is necessary to maintain central tolerance. We hope that nanoparticle encapsulated peptides could provide a possible pharmacological benefit for numerous autoimmune diseases in the future.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal